
In this bit of writing, I will build upon the last bit of writing about the Earth’s temperature increasing with information on the mechanisms that are trapping this heat. I will not discuss the role that Humans are playing in this process or the ramifications of the changes, just the process itself, the rest will be for a future bit of writing.
Carbon is the 15th most abundant element on earth. It exists in our atmosphere (Air) as Carbon Dioxide, in our Biosphere (Life) as the complex organic compounds that are the building blocks of life, in our Lithosphere (Land) as inorganic compounds in rocks and minerals, trapped gasses and fossil fuels and in our Hydrosphere (water) as trapped gasses and minerals.
How carbon travels between and within these spheres that make up our planet is defined as the carbon cycle and is vital to how are planet is able to sustain life.
Earth, the Sun and Energy Balance
The Earth receives energy, in the form of light energy from the sun and releases energy, in the form of Infrared Energy. In order to remain in temperature balance, the earth must release as much energy as it receives.
Of the 390 watts, on average, of Sunlight power per square meter that hits the earth, only 294 watts reaches the surface of the planet, the rest is reflected. Of the 294 watts that hits the planet, 40%, on average, of that reflects off into space from Ice caps, snow, deserts etc. the rest warms the surface and reflects back into our atmosphere as infrared energy instead of light. Water vapor, Carbon Dioxide and other greenhouse gasses reflect a certain amount of this heat back to the surface of the earth, without this “greenhouse” effect, the earth would likely be a big snowball.
The more greenhouse gasses in the atmosphere, the more heat is returned to the surface of the planet and the earth is unable to shed enough heat to maintain the temperature balance. When this happens, the temperature of the earth increases.
Greenhouse Gasses
The most abundant greenhouse gas in our atmosphere is water vapor, it is also the most self-adjusting of all the greenhouse gasses. The average Water molecule that evaporates spends about a week before it precipitates out and returns to earth. For this reason, water vapor is not considered a strong part of the global warming problem, to an extent, warmer air holds more water vapor, but it is not a major concern to most climate scientists.
Methane is a minor concern, that lasts on average five years in the atmosphere, Nitrous Oxide, Chlorofluorocarbons, Hydrofluorocarbons, ozone, etc.. are also adding to the problem, but not to the extent of Carbon Dioxide, which is the byproduct of combustion, respiration and decomposition.
The Carbon Cycle
The way that carbon moves through the departs of our environment is known as the Carbon Cycle. Our Atmosphere is comprised of the following:
- Nitrogen — 78 percent
- For the most part, an inert gas
- Oxygen — 21 percent
- Atmospheric Oxygen is what allows life on our planet
- Argon — 0.93 percent
- For the most part, an inert gas
- Carbon dioxide — 0.04 percent
- Plays a large part as a greenhouse gas
- Water Vapor – 0-3%
- as discussed earlier is the most influential greenhouse gas, but the earth self regulates this.
- Trace amounts of neon, helium, methane, krypton, ozone and hydrogen
- Varied but minor role as greenhouse gasses
The carbon dioxide levels in our atmosphere in human pre-industrial times has been measured at about 280 (ppm) parts per million, today we are at about 390ppm and we are climbing at about 2ppm per year.
Carbon is stored in our environment in several different ways:
- In the atmosphere it is stored as carbon dioxide
- In the oceans as Dissolved C02
- In plants and animals as complex organic compounds
- In the Earth’s crust as limestone and fossil fuels
Atmospheric Carbon, in the form of Carbon Dioxide is removed from the air through the process of plant’s photosynthesis.
Carbon Dioxide is returned to the atmosphere through the respiration of plants and animals, the decomposition of organic matter, the breakdown of limestone and other carbon-containing minerals, and the burning of organic matter and fossil fuels.
How do we measure Carbon Dioxide in the atmosphere?
One of the best locations on Earth to measure the composition of the gasses and particles in our atmosphere is the Mauna Loa Observatory in Hawaii due to its elevation and its distant location from high pollution areas where measurements would be skewed due top local conditions. They use a CO2 analyzer which uses infrared radiation passing through air in a cylinder, this gives us an accurate measurement.
NASA also uses satellite-mounted laser CO2 observatories for active measurements of the CO2 in the atmosphere as well as passive measurements that measure the light refraction inherent to CO2 concentration in the atmosphere.
We can also do direct measurements back 800,000 years by measuring CO2 quantities in ice cores as described in my earlier writing.
How do we determine Carbon Dioxide levels in the distant past?
For CO2 levels in the past, we can use proxies to determine levels back millions of years.
In fossilized leaves, we can examine the density of Stoma which are locations that absorb Carbon Dioxide for photosynthesis. In times of low CO2 concentration, the stoma are more densely packed in order to capture more air to filter out the needed CO2.
In soil, we can measure the isotope ratios of carbon-12 to carbon-13 as they break down at different levels over time, so absorbed Carbon Dioxide in soil particles can be accurately dated.
Measured conclusions
The data from actively measuring recent changes in Atmospheric Carbon Dioxide and using proxies to determine ancient levels of Carbon Dioxide shows long-term patterns of change over millions of years of variances in the CO2 in the Earth’s atmosphere, it also shows a significant change since the beginning of the human industrial age, a rate of increase not seen in the past. Both long- and short-term variances also coincide with changes in the Earth’s temperature.
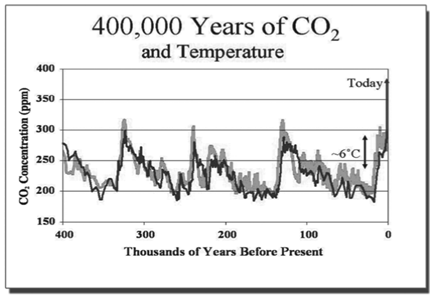
How do we tie the increased Carbon Dioxide in the atmosphere to global warming?
One of the cardinal rules in science is that correlation does not show causation. At this point, science shows that we have definitely measured an increase in temperature in our planet and also that we have definitely measured a higher level of CO2 in the atmosphere. When we plot these two long-term trends, the trendlines match, but that is not good enough as the resulting graph does not show causation. Using this logic, if we graphed the decrease in the world population of pirates next to the decrease in corded telephones used in our homes, we could say that phones are causing the pirate extinction.

We need to tie the temperature increase and CO2 increase together with scientific principles. Most scientific proofs are modeled in labs, but when dealing with a global environment, we need to break it down further.
One easy experiment beams infrared radiation through air chambers resembling the gasses in our atmosphere, as CO2 levels are adjusted, we can show that the increase in CO2 shows an increase in temperature at similar ratios to the historical ratios of CO2 and temperature in our atmosphere.
Computer models using huge mainframe servers are also used to predict the effects of an increase in CO2 in the atmosphere. All know variables are entered in and the predictive results can be seen over time. This method also allows us to adjust the variables to see what changes in our emissions could do over long term from both a positive and negative perspective.
In my next writing, I will explore the issue surrounding whether these changes are anthropomorphic, or human caused.
Sources:
https://nssdc.gsfc.nasa.gov/planetary/factsheet/earthfact.html
http://earthguide.ucsd.edu/virtualmuseum/climatechange1/05_1.shtml
https://www.esrl.noaa.gov/gmd/ccgg/about/co2_measurements.html
https://cdiac.ess-dive.lbl.gov/trends/co2/contents.htm
“The Earth’s Changing Climate” by Professor Richard Wolfson, The Teaching Companyhttps://pages.uoregon.edu/rdorsey/geo334/O-isotopes.html
Yes! Well done for doing your bit! I’ve been inspired to do something myself, so I have started a non-profit YT channel to try and help save the environment. If you could check it out, that would be much appreciated! https://www.youtube.com/channel/UC-g2DXCZtJyce9eVzUlkGKw
LikeLike